3a. If two water pipes are running side by side, one of which is hot while the other is cold, which will more likely be the “sweaty” one. Why is this? Use the kinetic model to give a complete answer.
- If two water pipes are running side by side, one hot and one cold, the “sweaty” one would be the cold pipe. This is due to the water vapor in the air condensing the cold water. Water vapor from the hot pipe contacts the cold pipe, and because cold air cannot hold as much water vapor as warm air, the temperature around the pipe drops and the air reaches its dew point; this results in the condensation that makes the pipe “sweaty” (https://www.superterry.com/fix-sweating-pipes/).
3b. Explain how the ancient Greek natural philosophers concluded that atoms must exist.
- Like we did in class, if you split something (in this case, a piece of paper) in half, there are two halves. If you continue this, eventually you come down to something you can either barely hold or barely see. With better technology and dedication, a person could further this process. The ancient Greek natural philosophers probably did something like this to a certain extent and came to an underlying conclusion that there is something that is made of all things and cannot be broken; something so small we cannot see it and something so strong we cannot break it. This indivisible piece was named “atoms”, after the Greek word “uncuttable” (https://plato.stanford.edu/entries/atomism-ancient/).
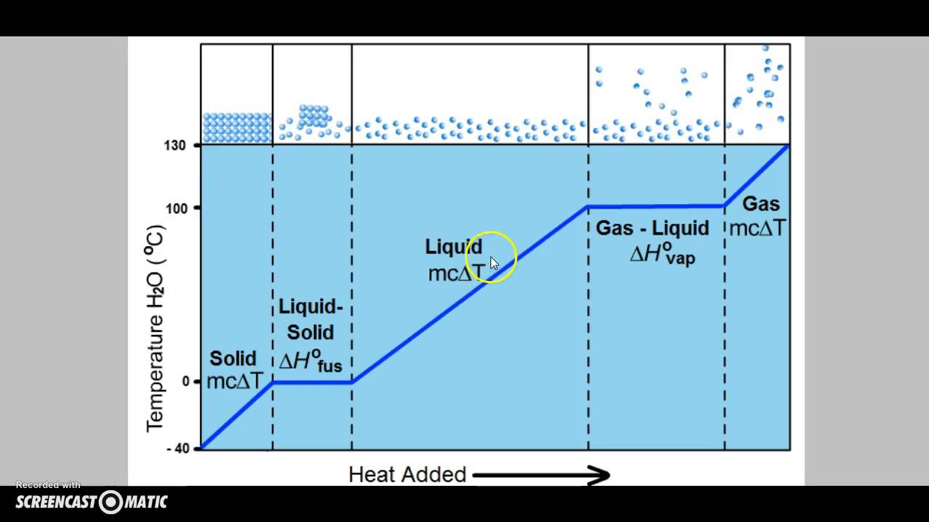
3c. Search the web and find a heating curve (i.e. a graph of temperature vs. heat that shows phase changes) for water or some other substance. Upload the graphic file as part of your answer. Explain its shape, especially the plateaus in the curve.
- Looking at the graph, if you read it from left to right, you’ll see that as we move up in temperature, the phase of water changes. At zero degrees Celsius and below, the water is frozen and kept in a solid form. Past zero, the water turns into a liquid-solid and eventually into a liquid. It stays a liquid until 100 degrees Celsius; then turning into a gas-liquid. After surpassing 100 degrees Celsius, the water officially becomes a gas. The reason there are horizontal lines in this graph is because there is a process of changing phases, it doesn’t simply just happen. During the horizontal times, the liquid bonds are being broken up into a different form, which takes time. (https://www.youtube.com/watch?v=7K2ZjWNpk_0) It should also be noted that, the temperature of a sample does not change during a phase change. During the melting of the ice, as long a little amount of ice is present then the temperature of the system will remain at zero degrees Celsius (https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_A_Molecular_Approach_(Tro)/11%3A_Liquids%2C_Solids%2C_and_Intermolecular_Forces/11.07%3A_Heating_Curve_for_Water).