3a.) If two water pipes are running side by side, one of which is hot while the other is cold, which will more likely be the “sweaty” one. Why is this? Use the kinetic model to give a complete answer.
When these two water pipes are running side-by-side the “sweaty” one would be the cold one. This is because the cold water is condensing because of the water vapor in the air. The water vapor in the pipe contacts the cold pipe; the water vapor molecules will slow down allowing them to get closer together. When the molecules slow down, the water droplets begin to form on the cold water pipe.
3b.) Explain how the ancient Greek natural philosophers concluded that atoms must exist.
The ancient Greek philosophers started off by first contemplating matter. Then they thought about the concept of splitting matter, and their concept was when you split something in half, it will always stay in two halves. They then realized that if you kept splitting the halves into smaller and smaller halves, there would then be apiece so small it would be invisible. The Greek philosophers called these pieces “atomos.”
3c.) Search the web and find a heating curve (i.e. a graph of temperature vs. heat that shows phase changes) for water or some other substance. Upload the graphic file as part of your answer. Explain its shape, especially the plateaus in the curve.
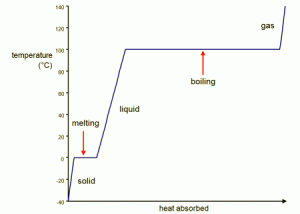
These plateaus in the line are where phase changes occur. During these phases the temperature stays consistent and the parts in the line that are changing are increases in heat.
https://www.kingarthurflour.com/learn/high-altitude-baking.html
This link describes cooking in different altitudes and explains the different things you need to do in order to cook things right. In higher elevations it takes linger to cook certain things.
https://wonderopolis.org/wonder/why-do-some-drinks-sweat
This link talks about how and why things sweat.
http://www.hyperhistory.com/online_n2/people_n2/science_n2/atomic_theory.html
This defines the theory behind matter and how the philosophers discovered it.
https://sciencing.com/easy-experiments-using-gas-laws-5506609.html
This website gives 3 demonstrations of experiments you can do at home.