3a) The colder pipe will be sweaty. The water vapor in the air causes the cold water to condense. When the water vapor comes in contact with the cold pipe, the water vapor molecules slow down and they get closer together, causing the vapor begins to turn into water droplets on the pipe. The hotter pipe isn’t sweaty because warm air holds more water vapor than cold air.
3b) When focusing on the concept of matter, the ancient Greek philosophers started with the notion that when you split matter in half, there will be two halves. From that concept, the philosophers found that at some point, after splitting halves into smaller halves, eventually a piece will become so small that it could not be separated in two pieces. They called this inseparable piece atamos, meaning uncuttable.
3c)
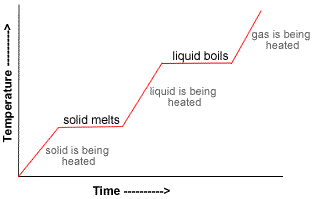 The lines that are increasing are the points where the temperature is rising as heat is added over time. The plateaus in the line are the points when a change in phase occurs, such as a solid to a liquid (boiling) or a liquid to a gas (evaporating). During this time of the phase changes, the heat does not increase the kinetic energy of the molecules, keeping the temperature constant.
The lines that are increasing are the points where the temperature is rising as heat is added over time. The plateaus in the line are the points when a change in phase occurs, such as a solid to a liquid (boiling) or a liquid to a gas (evaporating). During this time of the phase changes, the heat does not increase the kinetic energy of the molecules, keeping the temperature constant.



