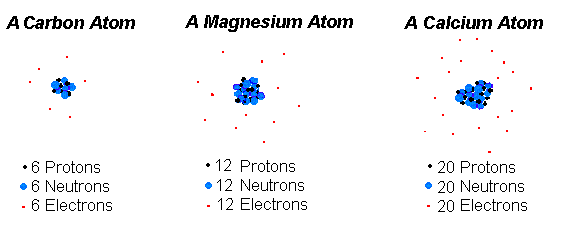
An atom a fundamental piece of matter. (Matter is anything that can be touched physically.) Everything in the universe (except energy) is made of matter, and, so, everything in the universe is made of atoms.
An atom itself is made up of three tiny kinds of particles called subatomic particles: protons, neutrons, and electrons. The protons and the neutrons make up the center of the atom called the nucleus and the electrons fly around above the nucleus in a small cloud. The electrons carry a negative charge and the protons carry a positive charge. In a normal (neutral) atom the number of protons and the number of electrons are equal. Often, but not always, the number of neutrons is the same, too.
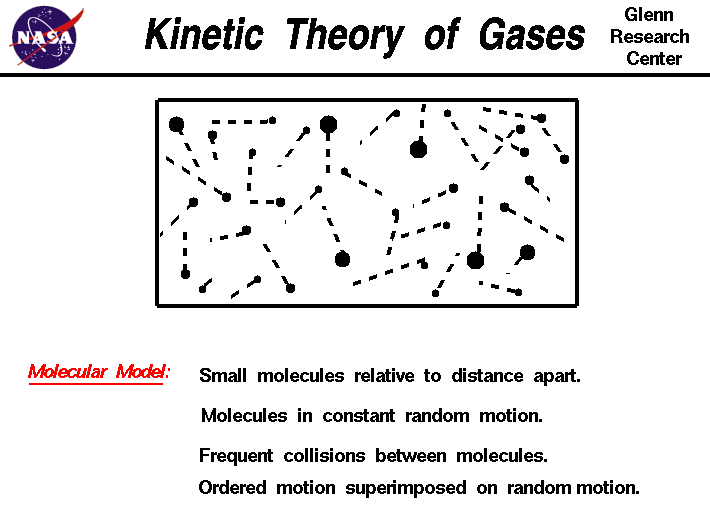
The model, called the kinetic theory of gases, assumes that the molecules are very small relative to the distance between molecules. The molecules are in constant, random motion and frequently collide with each other and with the walls of any container.
The individual molecules possess the standard physical properties of mass, momentum, and energy. The density of a gas is simply the sum of the mass of the molecules divided by the volume which the gas occupies. The pressure of a gas is a measure of the linear momentum of the molecules. As the gas molecules collide with the walls of a container, the molecules impart momentum to the walls, producing a force that can be measured. The force divided by the area is defined to be the pressure. The temperature of a gas is a measure of the mean kinetic energy of the gas. The molecules are in constant random motion, and there is an energy (mass x square of the velocity) associated with that motion. The higher the temperature, the greater the motion.
This site has plenty of video that explain each phase change and how it occurs. Sometimes a quick video is easier than trying to comprehend what is being taught. The line between the solid and liquid phases is a curve of all the freezing/melting points of the substance. The line between the liquid and gas phases is a curve of all the boiling points of the substance.
![[Image]](http://www.kentchemistry.com/images/links/matter/Phase.gif)
